OK I'm going to do my best to explain PH since its something that is for the most part greatly misunderstood and can be confusing to new growers and even experienced growers alike. This will explain why we need both ppm and PH meters to give us informed information about PH
This will be a simple guide leaving out a lot of information. So lets get started with a couple of definitions to help you understand.
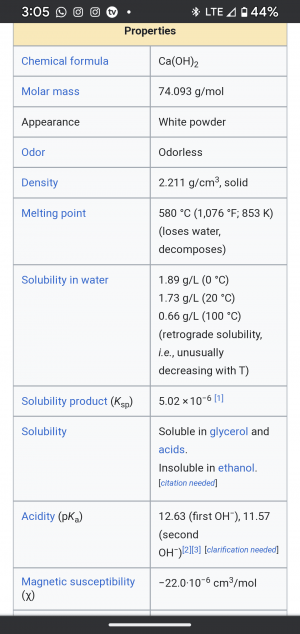
pH at it's core is a measure of the relative amount of free hydrogen and hydroxyl ions ratio. Ie H+ verses OH-. Quantified in molar mass
The pH scale is logarithmic and inversely indicates the activity of hydrogen ions in the solution.
So each, 0.1 increments is either double or half the concentration.... pH 7 to 8 would be a 10x fold increase in OH- ions
Whereas pH 7 to 5 would be a 100x fold increase of H+
pH=−log(H+)≈−log([H+])
Sulfuric hydrogen ion is a great acidic H+ anchor; Potassium hydroxide is a useful source for the hydroxyl OH- component. But both need to be tightly controlled. ( Contributed by @Franksta )
acidic H+ anchor; Potassium hydroxide is a useful source for the hydroxyl OH- component. But both need to be tightly controlled. ( Contributed by @Franksta )
So now we have a basic understanding of the difference lets get into some examples of source water and how alkalinity will affect PH.
When used in hydro it should have alkalinity (a buffer) added back to prevent wild PH swings. Any source of carbonates, bicarbonates, silicates or hydroxides will work to create alkalinity. Sources i would recommend would be calcium carbonate, potassium bicarbonate (commonly sold as PH up) and finally what i would consider the best option is potassium silicate as it is a source of potassium and silica which are both excellent for cannabis. When using RO or distilled you will want to add back some calcium and magnesium if your nutrients are not designed for RO/distilled water as that's usually what Ro filters are removing for the majority. But most cal/mag is in the form of nitrate and provides no alkalinity (buffering capacity) so adding one of the previously mentioned or other is still a must.
When used in soil this unstable PH is actually IMO beneficial if you have a pre buffered soil (which you should) This means the water will have no impact on the PH potential (more on this later) of the soil and will almost instantly be influenced by the soil to the take on the PH of the soil makeup. This is why i feel we do not need to be PHing our nutrient solution for soil grows (unlike soiless and hydro). The soil is what will adjust the PH of our nutrient solution.
Generally speaking the ratio's of nutrients we use will be acidic so when we get a buildup of nutrients we will almost always see PH drop. This is where you often hear ppl say flush the media. What this does is dilutes the dissolved elements and will remove some from the media in runoff. Another factor that contributes to PH changes are tge sources of nitrogen used. Ammoniacal nitrogen can cause PH in media to drop. This is due to the release of h+ ions from ion exchange. Whereas Nitrate nitrogen does the opposite and will cause PH in the media to rise when taken up by the plants.
Conversely a water source with high alkalinity can build up in the media and cause the PH potential of the soil to rise over time and in turn the PH of the water added to it. This is the reason we should look at the alkalinity of the water source not the PH as PH cannot measure the potential influence but rather only result.
Often in both circumstances its a good idea to flush the media to remove excess amount of available elements that may be affecting the PH negatively.
I'm gonna stop there and if anyone has questions i will do my best to answer them. If you have something you would like to add please do.
Aqua Man
This will be a simple guide leaving out a lot of information. So lets get started with a couple of definitions to help you understand.
What is PH?
PH is a measurement of how alkaline or acid a solution is based on measuring hydrogen ions. It tells us nothing more than the ratio of acidic to alkaline elements. It does not tell us how much of each the solution contains or the alkalinity of the water.pH at it's core is a measure of the relative amount of free hydrogen and hydroxyl ions ratio. Ie H+ verses OH-. Quantified in molar mass
The pH scale is logarithmic and inversely indicates the activity of hydrogen ions in the solution.
So each, 0.1 increments is either double or half the concentration.... pH 7 to 8 would be a 10x fold increase in OH- ions
Whereas pH 7 to 5 would be a 100x fold increase of H+
pH=−log(H+)≈−log([H+])
Sulfuric hydrogen ion is a great
What is alkalinity?
Alkalinity is the measurement of the waters buffering capacity (ability to neutralize acids). Its the total amount of carbonate and bicarbonate in the water that affects its ability to resist change to PH. If you know the alkalinity you can actually calculate the amount of acid of varying types needed to reach your target PH but we wont get into that.So now we have a basic understanding of the difference lets get into some examples of source water and how alkalinity will affect PH.
RO and Distilled water
Ro and Distilled water is very low in mineral content containing carbonate or bicarbonate sources, we know this because if we test the ppm its usually under 40 and as low as 0ppm. This means it has a very low alkalinity (ability to neutralize acids) and is easily influenced by anything added that's acidic. But likewise it does not contain acid and is easily influenced by anything added that's basic. This results in a very unstable PH that can be easily influenced by anything added or anything its added to. In hydro the ideal ppm of carbonate/bicarbonate sources to provide an adequate buffer will be 50-100ppm with 75ppm being the target. Less than this and PH may swing to fast and be unstable, more and it will not drift enough and will require too much acid that could affect nutrient ratio's negatively depending on the acid used. By adding alkalinity and then acid we provide a more stable PH because adding more of either will have less impact on the overall ratio of acidic to basic elementsWhen used in hydro it should have alkalinity (a buffer) added back to prevent wild PH swings. Any source of carbonates, bicarbonates, silicates or hydroxides will work to create alkalinity. Sources i would recommend would be calcium carbonate, potassium bicarbonate (commonly sold as PH up) and finally what i would consider the best option is potassium silicate as it is a source of potassium and silica which are both excellent for cannabis. When using RO or distilled you will want to add back some calcium and magnesium if your nutrients are not designed for RO/distilled water as that's usually what Ro filters are removing for the majority. But most cal/mag is in the form of nitrate and provides no alkalinity (buffering capacity) so adding one of the previously mentioned or other is still a must.
When used in soil this unstable PH is actually IMO beneficial if you have a pre buffered soil (which you should) This means the water will have no impact on the PH potential (more on this later) of the soil and will almost instantly be influenced by the soil to the take on the PH of the soil makeup. This is why i feel we do not need to be PHing our nutrient solution for soil grows (unlike soiless and hydro). The soil is what will adjust the PH of our nutrient solution.
Tap Water
OK we all know tap water varies a lot form place to place and I will explain the basics of how to determine if your tap water is suitable or not for use. First we want the PPM and second we want the makeup of that ppm if available. Generally speaking the majority of the PPM makeup will be calcium carbonate. This is used to buffer the water supply and prevent acidic conditions that erode the coatings and will break down piping and leach them into the water supply such a lead (Flint Michigan ring a bell?) So we can generally assume the majority of the PPM in tap water is likely calcium carbonate but also some others like magnesium, sulfur, phospahte, iron etc. So if you have a ppm of 100-200ppm you can assume roughly 50-75% of that is calcium carbonate. Remember our target is 75ppm carbonate/bicarbonate sources to provide an ideal alkalinity (hope we are starting to see how import alkalinity is and we can't just go by PH) Now there are some cases when some sodium may be used such as sodium bicarbonate aka baking soda (can also be used as a buffer in a pinch but not recommended as a long term option) so we can google our local water report and see the makeup of the ppm in the water.Soil PH potential
Now when we buy a prebufferd soil like most of the ones we use they come "Prebuffered" (alkalinity adjusted) what does this mean? This means the company has added amendments that when water is added the resulting PH of the water in the soil will be in a favorable range for growing our plants. Often times peat is used to lower PH and lime is used to raise PH in these soils. Just like in water we want to control the alkalinity (buffering capacity) of the soil to have a stable PH that is not easily influenced by adding things such as nutrients or other. Unlike hydro and soiless where we control the alkalinity (buffering capacity) of the water by adding it directly to the water it is applied to the soil. Which brings me back to my point of we don't need to PH our nutrient solution in soil because the soil provides the buffering and will adjust the PH. Now things like lime and peat break down slowly over time and only soluble elements will impact PH so this is how they control the PH in soil over long periods of time, because it breaks down slowly and only a small amount is soluble at a time its unlikely after a grow it has been depleted. But if we are reusing the soil we should be looking at re amending the buffering capacity before using again to ensure there is enough to last through the next grow. Often times farmers will do this once a year before seeding crops.Effects of nutrients and source water on PH
First the PH down acids we use tend to break down much faster than the alkaline sources we use in both hydro and soil. This is one reason we see a hydro systems PH generally rise over time unless something is creating more acid like decaying roots in which case we may actually see PH going down. Typically a PH increase of 0.2 in a 24 hr period is desirable and by adjusting the alkalinity we can control the PH drift. In the case of soil the acids used to bring PH down before feeding break down quickly and the alkaline and acidic buffer we have created minimizes the impact so they are really of not much benefit and have virtually no impact on long term PH potential of the soil. This is why we can't use them to lower high soil PH once we have an alkaline source buildup. However in hydro and coco PHing the nutrient solution is important because unlike soil there is not an adequate buffer established although in coco it is possible to do so.Generally speaking the ratio's of nutrients we use will be acidic so when we get a buildup of nutrients we will almost always see PH drop. This is where you often hear ppl say flush the media. What this does is dilutes the dissolved elements and will remove some from the media in runoff. Another factor that contributes to PH changes are tge sources of nitrogen used. Ammoniacal nitrogen can cause PH in media to drop. This is due to the release of h+ ions from ion exchange. Whereas Nitrate nitrogen does the opposite and will cause PH in the media to rise when taken up by the plants.
Conversely a water source with high alkalinity can build up in the media and cause the PH potential of the soil to rise over time and in turn the PH of the water added to it. This is the reason we should look at the alkalinity of the water source not the PH as PH cannot measure the potential influence but rather only result.
Often in both circumstances its a good idea to flush the media to remove excess amount of available elements that may be affecting the PH negatively.
I'm gonna stop there and if anyone has questions i will do my best to answer them. If you have something you would like to add please do.
Aqua Man
Last edited: