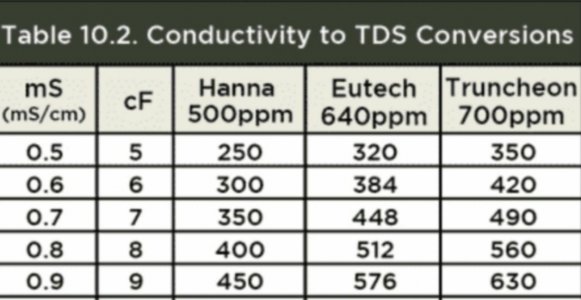
I think this is worth fleshing out. We can take this to your PH thread if you like.
First, Potassium Silicates such as AgSil 16H:
Do potassium silicates contain “less available” silicon?
When you dissolve a potassium silicate at high concentration, it forms silicate oligomers. These are large silicon chains that get stabilized in basic solutions because of their high negative charge. This is why you can create highly concentrated potassium silicate solutions in basic pH. As a matter of fact, making the solutions more basic with added potassium hydroxide often enhances the solubility of potassium silicate solids like AgSil16H (see
here for a procedure on how to do this). However, when the molar concentration decreases the silicate hydrolyzes into monomeric silicate anions.

Original background image taken from
here. To create a monomeric solution you need high pH and low concentration. Then you lower the pH to get to monosilicic acid.
When potassium silicate is diluted in nutrient solutions, this is exactly what happens. The reduction in concentration hydrolyzes the Silicates into monomers. If the solution pH is
then lowered, the final form present will be monosilicic acid. If you properly prepare a nutrient solution with potassium silicate, the end form will be monosilicic acid, the form that is mostly available to plants.
It is a misconception that potassium silicates are somehow less “plant available”. They end up producing monosilicic acid and being perfectly available, when used properly.
OK, so what.
Concentrated soluble potassium silicate has a pH of 12.7, which
increases the pH
PH Down, when used in conjunction does in fact produce mono-silicic acid as a byproduct which is beneficial to the plant and readily available for uptake. This is the stuff that helps with strength primarily in cell walls.
I'm taking bits and pieces from several spots here, but it is all pretty well fleshed out
and across several other blog posts and videos. If you have a few hours to spend, it's a really great place to spend it.
This is the author:
My name is Dr. Daniel Fernandez. I have a bachelor’s degree in chemistry and a masters and Ph.D. in nanoscience and nanotechnology. I have a strong interest in plants and hydroponic crops.
And he goes on to show how to set up the best buffer with the cheapest inputs and Agsil 16H is on the very top of every list he has.
I personally have not used it yet, I'm still working thru another PS product. When I run out I am switching to Agsil.
So, bottom line, put in the Agsil to the RO water (not in your res unless just starting out) which will send the PH way high.
Let it stir for 24 hours.
Add in PH down to get you to 5.8
You now have both an impressive buffer and plant available silicon. At least according to the good doctor. He seems pretty credible to me at least.